- For the first time in surgical history, a 3D-printed ceramic subperiosteal jaw implant was placed in a patient as part of the INKplant project
- Together, the high precision of Lithoz LCM technology and the design freedom of 3D printing made this ceramic jaw implant „under the periosteum“ possible in a world first
- This innovation, which requires only one procedure, is estimated to reduce healing time after such oral surgery by 75%
- Lithoz once again demonstrates the enormous innovation potential of ceramic 3D printing with this research contribution
2nd July 2024: Vienna / Linz, Austria. In a major breakthrough for medical applications, a 3D-printed ceramic subperiosteal jaw implant was successfully placed in a patient for the first time ever. Fabricated in Austria, this innovation marks a significant advancement for medicine and is part of the EU-funded INKplant project made up of 19 interdisciplinary partners, led by Profactor GmbH. The project aims to create 3D-printed patient-specific implants to treat various pathologies affecting the elderly.

Lithoz, one of the partners in the project, has been researching the optimal fusion of various biomaterials with the advantages of 3D printing since 2021. The implant was developed with and built by Austrian ceramic 3D printing specialist Lithoz to address the issue of atrophic jaws, a common problem in older patients. After the loss of teeth, the jawbone disappears as well, resulting in atrophic jaws and rendering the use of dentures impossible. With severe atrophy, conventional dental implants require additional lengthy operations to graft new bone to anchor the implants. Such operations are difficult for elderly patients who cannot undergo bone grafting due to health issues.
A patient at Kepler University Hospital, who had lost multiple dental implants and bone grafts in the past due to his compromised health, was unable to receive further conventional surgical strategies as a result of significant scarring and thus received the new implant as a compassionate use case. Made of biocompatible high-strength zirconia using Lithoz LCM technology, the implant did not require any bone augmentation and required only one procedure, reducing healing time by an estimated 75% and avoiding excess trauma for the patient. Thanks to this synergetic innovation in design and material, all the necessary surgical procedures were completed in a single operation.
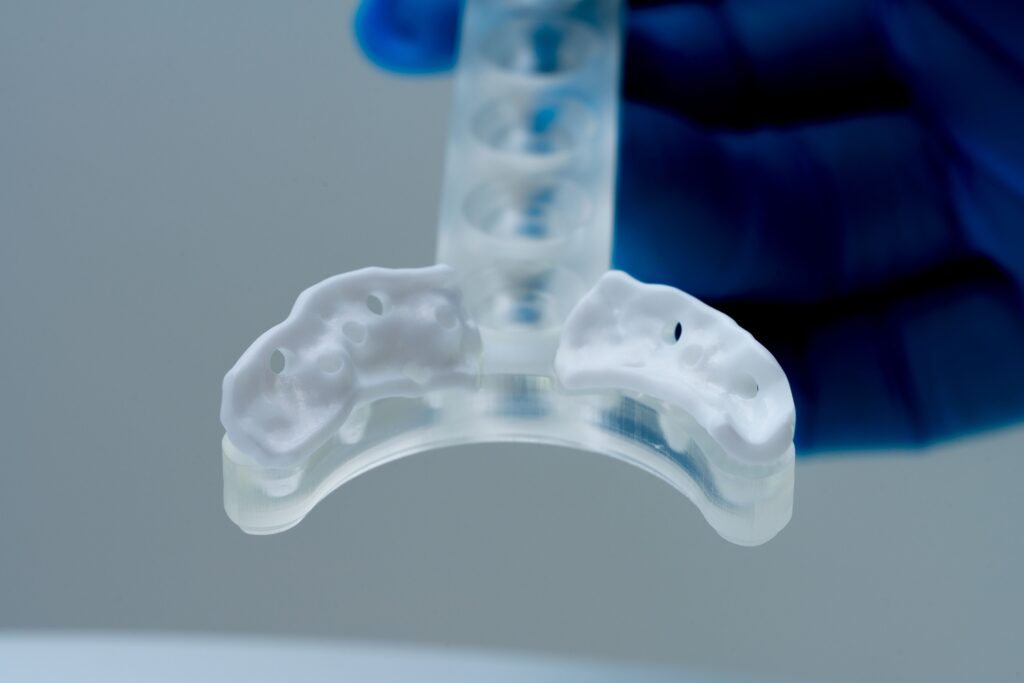
The surgery, led by DDr. Christoph Staudigl, was a successful world first use of a ceramic subperiosteal jaw implant in a compassionate use case on a patient. Despite some expected wound healing issues after surgery, the superior soft tissue compatibility of zirconia compared to titanium played its role spectacularly. The implant showed clinical stability after 60 days, representing a decisive breakthrough for the treatment of severely atrophic jaws.
The design of the customized implant was pioneered by the Centre for Medical Physics and Biomedical Engineering at the Medical University of Vienna in collaboration with DDr. Staudigl. During the design process, BTI Biotechnology Institute (Vitoria-Gasteiz, Spain) and BioMed Centre Innovation GmbH (Bayreuth, Germany) also contributed significantly with their expertise. The implant will be patented and adopted as a medical device by BioMed Centre spin-off Agensmed GmbH and will be manufactured using Lithoz 3D printers. A clinical trial is being prepared to systematically validate its efficacy.
Funding

The project INKplant has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 953134. www.inkplant.eu

About Lithoz
Lithoz is the world and technology leader for high-performance ceramic materials and 3D printers. Founded in 2011, Lithoz is committed to breaking the boundaries of ceramic production and supporting customers in expanding the manufacturing opportunities for the ceramic industry. The company has an export share of almost 100%, more than 150 employees and 4 different sites worldwide. Since 2016, Lithoz has also been ISO 9001:2015 certified.
Lithoz Contact: Alice Elt +43 660 1563231 / aelt@lithoz.com
About Agensmed:
Agensmed GmbH is a medical device manufacturer located in Bayreuth, Germany with a focus on additive manufacture of bone. The company prints operational models, veterinary bone substitute and human implants for bone treatment. By applying innovative AM technologies and design strategies it meets the requirement for individual and regenerative treatments realizing improved and sustainable healing. It is a spin-off from INKplant partner BioMed Centre Innovation GmbH and realizes the subperiosteal implant and other project results as medical devices registered for the EU under MDR.
Agensmed GmbH contact: Daniel Seitz / d.seitz@agensmed.com
About BTI Biotechnology Institute
BTI Biotechnology Institute I MAS D, S.L. is a Spanish private R&D centre and part of BTI Group. BTI Biotechnology Institute was founded in 1999 and today is one of the leading companies in implantology and oral rehabilitation, as well as an international scientific benchmark in the application of regenerative therapies in different areas of medicine including oral and maxillofacial surgery. BTI has a multidisciplinary professional team made up of more than 300 people, of which 20% is dedicated to R&D.
BTI Contact: Mohammad Alkhraisat +34 945 160653 / mohammad.hamdan@bti-implant.es
About Kepler University Hospital
With approximately 7,000 employees and around 1,800 beds, Kepler University Hospital is Austria’s second-largest hospital, encompassing numerous medical specialties as well as experts from all health professions. The Med Campus site offers the full range of surgical, conservative, and diagnostic services. It also houses a comprehensive center of excellence for women’s, children’s, and adolescent medicine. Over the past decades, the Neuromed Campus has established itself as an international center for neuromedicine. Here, patients with diseases of the brain, spinal cord, nervous system, and mental illnesses are treated.
Kepler University Hospital Contact: Brigitte Buberl +43 5 7680 83 – 1400 / brigitte.buberl@kepleruniklinikum.at
About Medical University of Vienna
The Medical University of Vienna is renowned as one of Europe’s most venerable institutions for medical education and research. With nearly 8,000 students, it holds the title of the largest medical training facility in German-speaking nations. Its extensive framework encompasses over 6,000 personnel, 30 departments, two clinical institutes, and 12 medical theories centres, along with numerous state-of-the-art laboratories. Notably, among its centres, the Centre for Medical Physics and Biomedical Engineering houses a large infrastructure and research laboratory dedicated to additive manufacturing for various areas of medical research.
Medical University Contact: Francesco Moscato +43 1 40400 39830 / francesco.moscato@meduniwien.ac.at
About Profactor
Profactor is an Austrian research company that develops new methods for integrated production technologies. The company focuses on functional surfaces and nanostructures, additive manufacturing, robotics, and machine vision. With a long record of participation in national and international funded projects, Profactor is the coordinator of INKplant. Founded in 1995, their technological developments have always strived for efficiency and sustainability and work to benefit society.
Profactor Contact: Michael Kainz / michael.kainz@profactor.at

